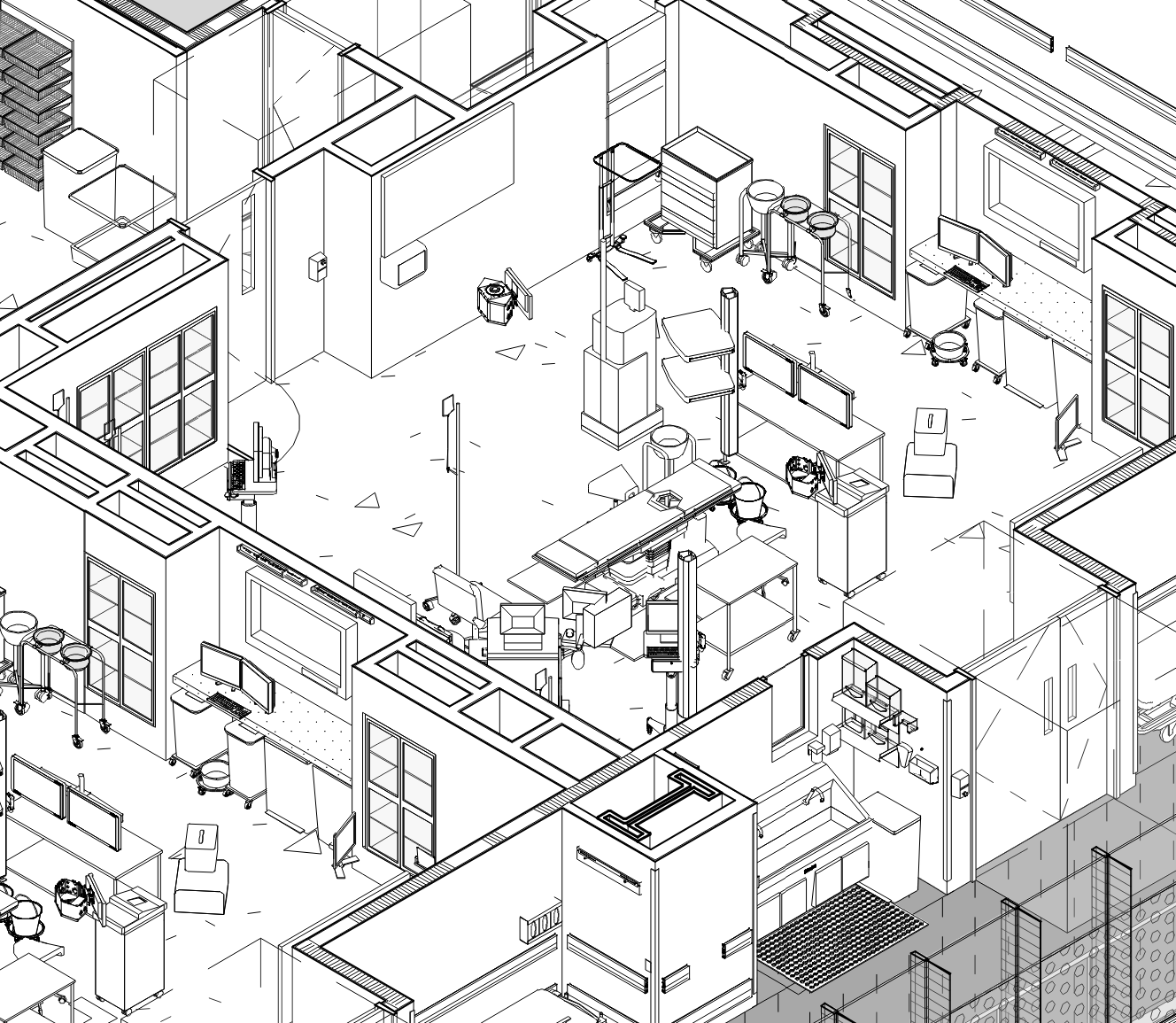
Services
MEQA's Services and Deliverables are designed to comprehensively develop and manage the Medical Equipment Scope of work within a Healthcare Design project. However, we understand these efforts can have many facets and circumstances and we're happy to discuss a set of custom deliverables to meet the needs of your Project. One, all or a blend of approaches- we'd love the opportunity to discuss teaming with you!
Master & Validation Level
Planning and Budget Development
It’s never too early for specifics when planning expansions and improvements within a Healthcare system or campus, and specificity is what you can expect in the documentation that comprises our Master Planning and Validation level equipment packages. More than referencing documents from previous, similar projects, MEQA builds and documents medical equipment designs that not only reflect the high level goals within the Master Plan, but also the Vendor and Operational preferences specific to the site. Current and future care models, equipment re-use, budgetary contingencies, preferred Vendors and contract based pricing for high dollar-value systems are just a few of the aspects built in to all of our validation studies. All items determined to fall within the Medical Equipment scope are detailed in full, supported by Manufacturer provided product specifications and anchorage details and summarized with an Approach Narrative for easy integration into your validation study submittal presentation.
Design & Construction
Planning and Coordination
MEQA’s proactive approach to Equipment Planning is a direct response to the experiences we’ve seen when medical equipment is neglected on any of its many fronts. We understand how our role informs the designs and commitments of our Team, and we’re eager to lead the dedicated efforts required to develop equipment sets that reflect the goals of all stake holders.
Equipment review meetings are more than where we come together to say yes or no to what should be in a room. Each item has the potential to improve some aspect of the project or the reality for the people who will work and be cared for in it. With MEQA, client meetings are opportunities to build confidence, share ideas that lead to improved outcomes and drive decision making. Because of its position as the industry standard for Equipment Planning, MEQA uses the Attainia software platform to develop, update and disseminate medical equipment information to its customers and partners. MEQA is Attainia certified and we’re proud to note ourselves as some of the longest standing users of this innovative planning tool.
With such a versatile planning platform at our disposal, you can expect all your budgets, lists, cut sheets, specifications and site specific details to be developed accurately and quickly. And, we’re dedicated to teaching how to most effectively use our reports; we’ll suggest queries to get to specific subsets of information, or we’ll augment standard reports to help you complete your commitments with greater efficiency.
Equipment Location Drawing
No Medical Equipment submittal package is complete without location drawings. Our AutoCAD drawings are living documents that can be drawn to be a one sheet reference for Medical Equipment item location, MEP/S coordination, Clearance Requirements, site specific Vendor Drawings, key IT/IS devices and Furniture. As the project moves to Procurement and Activation, we continue to update and augment so that Medical Equipment Location Drawings also serve Movers, Installers and Staff who are occupying the building. MEQA offers both full Equipment Location Drawing services as well as collaboration and review of Drawings, should an in-house approach for graphics be adopted.
Procurement Assistance & Support
MEQA’s procurement strategy is anchored in establishing integrated, persistent, working relationships with all stakeholders in the Procurement and Delivery phases of a project. In conjunction with the Owner’s purchasing team, MEQA plugs in to established process and resources, re-doubling the effort to attain the most competitive terms and pricing for Owner preferred Medical Equipment. We’ve witnessed first hand the value of similar, integrative strategies with our Construction partners and prioritize aggressive adherence to the Construction Schedule in our dialog with the Vendor base and minimal response and solution times when questions or challenges materialize. Our long standing in California’s Healthcare Design market affords us many strong relationships with the industry’s most popular Vendors, and we leverage those relationships to help ensure that Vendors and product are where and when we need them. When it’s time to Procure, MEQA will facilitate a smooth flow of information and timely delivery and installation of product from Design Sign Off through Reconciliation and all the phases in between.
Inventory, Relocation &
Installation Management
MEQA’s approach to existing Medical Equipment is to perceive and manage it as though it were new. While on site we will prioritize speed and efficiency and make every effort to minimize the impact of our presence in the Care Environment. We work around Staff schedules so Clinicians can focus on patient care and do our utmost to ensure patients aren’t distracted or given cause for concern.
At the same time, when we visit we are in fact there for the details. We understand that Equipment re-use is more than simply an approach to reduce budgets and that Architects and Engineers need the same level of detail for existing equipment as they do for new purchases. To that end, we aim to collect key pieces of information during our visit, including Make and Model, Bio-Med and Facilities Management asset numbers and field pictures to aid us in determining remaining useful life.
With that information in hand we’re positioned to acquire Manufacturer product data for existing items so that we can accurately inform our Engineering Peers, we have visual references to aid us in making re-use determinations with Staff, and we are speaking the language of the teams who run and maintain the building and its equipment so that we can integrate their perspective on whether an item should be replaced. We also explore costs that can be associated with keeping a legacy item in service like extended maintenance agreements or hardware and software upgrades.